Atherosclerotic Plaque as a Lymphoid Organ
An introduction
Cardiovascular diseases challenge global health. Traditional risk factors are key, but cardioimmunology shows the immune system's complex role in these diseases, especially in atherosclerosis, now seen as a chronic inflammatory condition. Findings like structured immune cells, termed tertiary lymphoid organs (TLOs), in atherosclerotic plaques, indicate the immune system's active role in disease progression and potential coronary events triggers.
Tertiary Lymphoid Organs (TLOs) in Plaques
The discovery of TLOs, specifically referred to as Plaque Tertiary Lymphoid Organs (PTLOs) in the context of atherosclerosis, offers clear evidence of local adaptive immune responses occurring directly within the arterial wall. These structures are not encapsulated but show some level of organization, containing collections of lymphoid cells. Their formation is typically linked to chronic inflammation. The identification of PTLOs in human carotid atherosclerotic plaques using methods like spatially resolved single-cell transcriptome mapping, H&E staining, and immunofluorescence confirms their presence and classification as TLOs within these lesions.
Cellular Composition of PTLOs
PTLOs are marked by a notable accumulation of lymphoid cells, with prominent populations of T cells and B cells. Compared to other areas within the plaque, PTLOs tend to have a higher number of cells from the B lineage, including plasma cells. Analysis of human coronary atherosclerotic plaques shows a substantial proportion of alpha-beta T cells. Many of these T cells found in plaques are not naive but are antigen-experienced memory T cells, primed to respond quickly upon re-exposure to an antigen.
The Critical Role of T Cells
T cells found in plaques show signs of clonal expansion, suggesting they have been activated by specific antigens present in the plaque environment. Single-cell RNA sequencing (scRNA-seq) analysis of human atherosclerotic plaques has revealed various T cell subsets. Importantly, studies have found that plaque T cells, especially CD8 T cells, can be specific for antigens from common viruses like Influenza (Flu), Cytomegalovirus (CMV), and Epstein-Barr virus (EBV). This finding implies that ongoing or repeated viral infections might help maintain persistent inflammation by activating these resident T cells.
Furthermore, the concept of molecular mimicry comes into play, where viral structures are similar enough to self-proteins that an immune response initially targeting the virus mistakenly attacks the body's own tissues. Experimental data support this, showing that T cells isolated from plaques can react to both viral and self-derived targets, suggesting that long-term viral exposure could initiate or sustain an autoimmune element in atherosclerosis.
Beyond promoting inflammation, plaque T cells also influence the physical characteristics of the plaque. In more advanced atherosclerotic lesions, there is an increased presence of T cells that produce Amphiregulin (AREG), a cytokine known for its pro-fibrotic effects. AREG from plaque T cells can affect smooth muscle cells (SMCs) and macrophages, potentially contributing to the development of fibrosis and structural changes seen in later stages of plaques. Certain T cell types, like CD8 Tem2 cells, display characteristics linked to inflammation and cell killing in advanced disease.
B Cells and Local Antibody Production
B cells are essential components of plaque TLOs and the atherosclerotic lesion as a whole. While their role is complex, their structured arrangement in PTLOs is particularly significant. PTLOs can contain B cells that express markers of germinal centers, indicating local processes of maturation, proliferation, and differentiation into plasma cells capable of producing antibodies within the arterial wall, mirroring processes observed in some autoimmune diseases. Specific chemokine signaling, such as the CXCL13-CXCR5 pathway and others like CXCL12, CCL19, and CCL21, are associated with the organization of B cells and the formation of TLOs.
The local generation of antibodies, particularly IgG antibodies, by plasma cells found in or moving into PTLOs, seems to have important consequences. These locally produced IgG antibodies can interact with Fcγ receptors (FcγRs) present on immune cells like macrophages. Specifically, binding to FCGR3A (CD16), found on macrophages, can mediate the effects of these antibodies. Studies have shown a correlation between the expression of IGHG4 in plasma cells and FCGR3A in macrophages within atherosclerotic plaques. High levels of macrophage FCGR3A have been observed in symptomatic carotid plaques, suggesting a possible link to plaque instability. IgG antibodies can form immune complexes with oxidized LDL (oxLDL), which can then trigger inflammatory responses in macrophages. Therefore, the differentiation of B cells and the local production of IgG within PTLOs likely play a role in plaque instability by boosting macrophage activation.
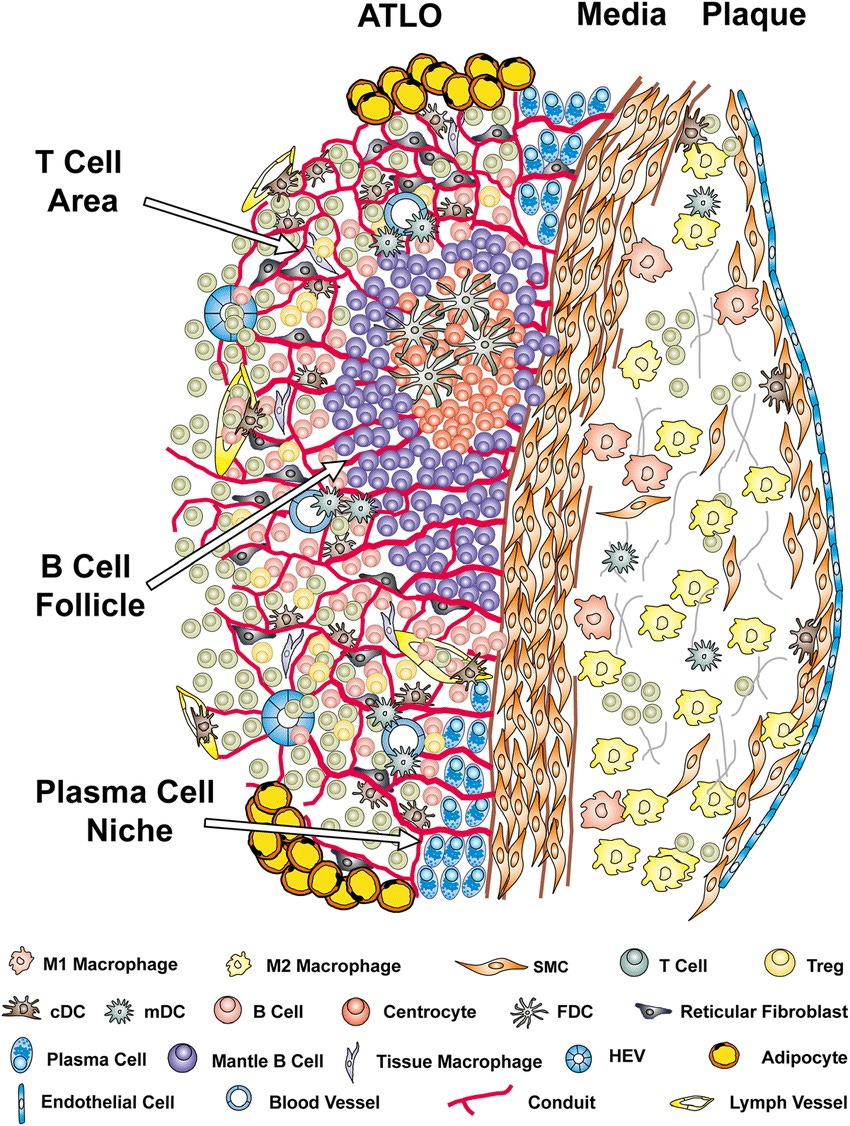
Well-structured artery tertiary lymphoid organs (ATLOs) arise adjacent to advanced atherosclerotic plaques in the abdominal aorta of aged Apoe-/-mice.
Cellularity, structures, and the territoriality of ATLO neogenesis indicate comprehensive T-and B-cell responses toward unknown arterial wall-derived autoantigens
Advanced ATLO stages are characterized by separate T-cell areas, activated B-cell follicles, and plasma cell niches in the periphery.
Autoantigen presentation is indicated by the presence of follicular dendritic cells (FDCs) in activated germinal centers; the abundance of cDCs and monocyte-derived DCs (mDCs) in T-cell areas;
B-cell affinity maturation is indicated by multiple centroblasts and their progeny in B-cell follicles; a balance between pro-and anti-inflammatory and T cells is indicated by multiple effector T cells and regulatory T cells (Tregs);
newly formed conduits may maintain chemokine gradients and possibly guide autoantigen diffusion from the diseased arterial wall toward ATLO antigen-presenting cells. HEV indicates high endothelial venule; and SMC, smooth muscle cell.
TLOs, Lymphangiogenesis, and Plaque Instability
The formation and function of TLOs are also connected to lymphangiogenesis, the growth of new lymphatic vessels. Research in transplant arteriosclerosis, a related vascular condition, has demonstrated a link between the growth of lymphatic vessels and the formation of TLOs, with early interventions targeting lymphangiogenesis showing potential long-term benefits. In atherosclerosis, genes related to lymphangiogenic chemokines are associated with the development of PTLOs, pointing to an interaction between local lymphatic networks, the movement of immune cells, and the organization of TLOs. The density of CD31+ microvessels is higher in areas with PTLOs compared to areas without. PTLOs themselves have been shown to be associated with symptomatic carotid atherosclerosis.
Implications for Therapeutics
The presence of TLOs in atherosclerotic plaques fundamentally alters our understanding, highlighting atherosclerosis as a disease partly driven by organized, local adaptive immune responses. These structures act as central points for interactions and activation among immune cells, and potentially for the local production of autoantibodies. Understanding the detailed roles of T cells in inflammation and fibrosis, and the complex activities of B cells, particularly the pro-inflammatory capacity of locally produced IgG antibodies binding to FcγRs on macrophages, is essential for grasping plaque progression and the associated risk of clinical events.
The in-depth knowledge of PTLOs and the specific immune cell populations within them provides promising avenues for identifying new targets for treatment. The key challenge is to develop therapies that can specifically target these local immune structures and pathways without broadly suppressing the immune system. The rapid progress in cardioimmunology, supported by technologies like single-cell and spatial transcriptomics, continues to reveal the extensive involvement of the immune system in atherosclerosis, paving the way for more targeted and effective strategies to prevent and treat this widespread disease.
#Atherosclerosis, #Cardioimmunology, #Immunology, #TertiaryLymphoidOrgans, #VascularBiology, #Inflammation, #TCells, #BCells, #Macrophages, #SingleCellRNAseq, #SpatialTranscriptomics, #CardiovascularDisease